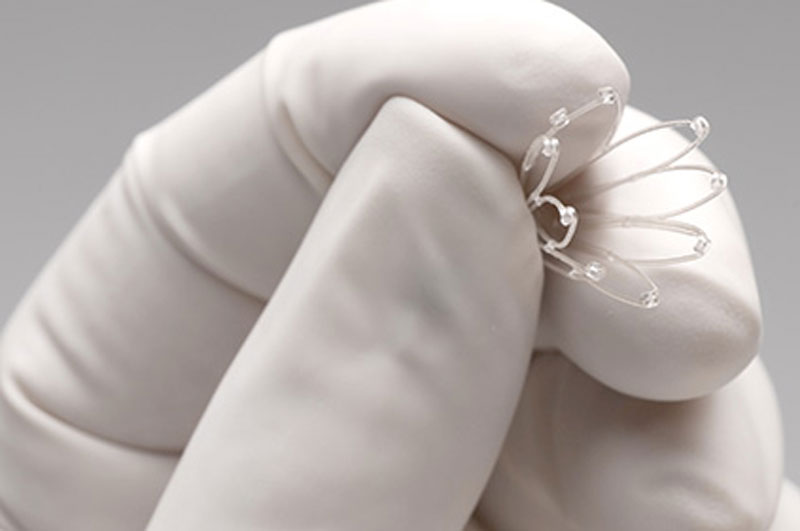
What Are PROPEL Sinus Implants?
How Does a PROPEL Sinus Implant Work?
Who Is a Good Candidate for a PROPEL Sinus Implant?
Patients who have not found sufficient relief from nasal polyps or chronic sinusitis with non-surgical treatments may be candidates for the PROPEL sinus implant. The implant may be suggested for:
- Chronic sinusitis of more than four months
- History of nasal polyps
- Inadequate relief from conservative therapies such as oral steroids or steroid rinses
Benefits of a PROPEL Sinus Implant
Combined with FESS surgery, the PROPEL Sinus Implant offers multiple benefits, such as:
- Maintained widening of the sinus passages post-surgery
- Improved surgical success rate
- Reduction of post-surgical inflammation and scarring
- Reduced chances for additional surgery
- Decreased need for sinus medications such as oral steroids or steroid rinses
What are the Risks of a PROPEL Sinus Implant?
Before considering the PROPEL sinus implant, patients must undergo a consultation and examination to determine their candidacy for the treatment. Those who are suspected or confirmed to have an allergy to mometasone furoate or any of the implant’s ingredients, including glycolide, lactide, and caprolactone copolymers, are not suitable candidates.
Similar to other implanted devices, the PROPEL sinus implant may cause side effects, such as a foreign body reaction, which may require further treatment. Although rare, there is also a possibility of developing toxic shock syndrome, a potential complication of any surgical procedure.
The most common side effects reported during PROPEL clinical trials include:
- Headache
- Nose bleeds
- Lightheadedness
- Sinusitis
- Nasal polyps
- Respiratory infection
- Asthma attacks
- Nausea
- Eyelid swelling
How Long Will the PROPEL Sinus Implant Results Last?
Clinical trials have demonstrated that PROPEL sinus implants provide significant improvement in patient outcomes following FESS surgery. The implant has been clinically proven to be effective in:
- Scarring reduction by up to 70%
- Reducing the need for follow-up interventions, including steroid medication, by up to 35%
- Reduction of polyp formation by up to 46%